Meningiomas are the most common primary central nervous system tumor. They are usually benign, but approximately 20% of meningiomas unexpectedly become clinically aggressive after gross total resection. While surgery and radiation are usually effective for benign tumors, there is no standard of care for more aggressive disease. Unfortunately15% to 35% of all meningiomas are high-grade (aggressive), inoperable and/or radiation resistant. While pediatric meningiomas are rare, they exhibit increased high-grade features and predilection for atypical sites, resulting in challenging surgical resection (Ravanpay et al., 2018). Radiotherapy is reserved for patients that do not respond to surgery, but the benefits of it are paradoxical. Cranial irradiation is a risk factor for meningioma development and, for pediatric cases, radiation delivered to the developing brain results in significant morbidity without benefits of long-term cure. These observations highlight the need for novel approaches to treat meningiomas.
Omics has allowed the understanding of pathogenesis and advancement in therapies for cancer. Our group has recently molecularly characterized this subgroup (Parada et al., 2020). In meningiomas, omics data has revealed unique molecular heterogeneity between pediatric and adult meningiomas, suggesting that personalized therapies may be an ideal treatment approach. Single-cell sequencing data has revealed a detailed landscape and allowed the identification of heterogeneous cancer transcriptomes, oncogenic activities and distinct microenvironmental patterns ripe for targeting. Precision targeted therapies for genetically heterogeneous meningiomas are certainly possible but lacking.
Kinase inhibitors (KIs) play an important role in cancer treatment. Using well-established clinical samples from our biorepository, we have described kinase dysregulation as a mechanism of
aggressiveness/progression in meningiomas (Parada et al, 2018, Parada et al., 2020). Multi-omics data suggest meningiomas may respond to cell cycle KIs. Targeted KIs are considered “smart drugs” when compared to chemotherapy/radiation because they target the tumor and spare normal tissue. Leveraging this treatment approach may be protective to the brain and spinal cord, especially in children.
Cancer KI trials suffer from non-genotyped subjects, delaying implementation of therapeutics into clinics. Personalized KIs, which can simultaneously inhibit parallel pathways or nodes in a single cascade, may improve efficacy, reduce drug resistance, and accelerate clinical implementation. Artificial intelligence (AI) aids in the interpretation of data-intensive assays. We believe the integration of multi-omic data with KI validation holds the potential to reveal appropriate strategies for the treatment of meningiomas.
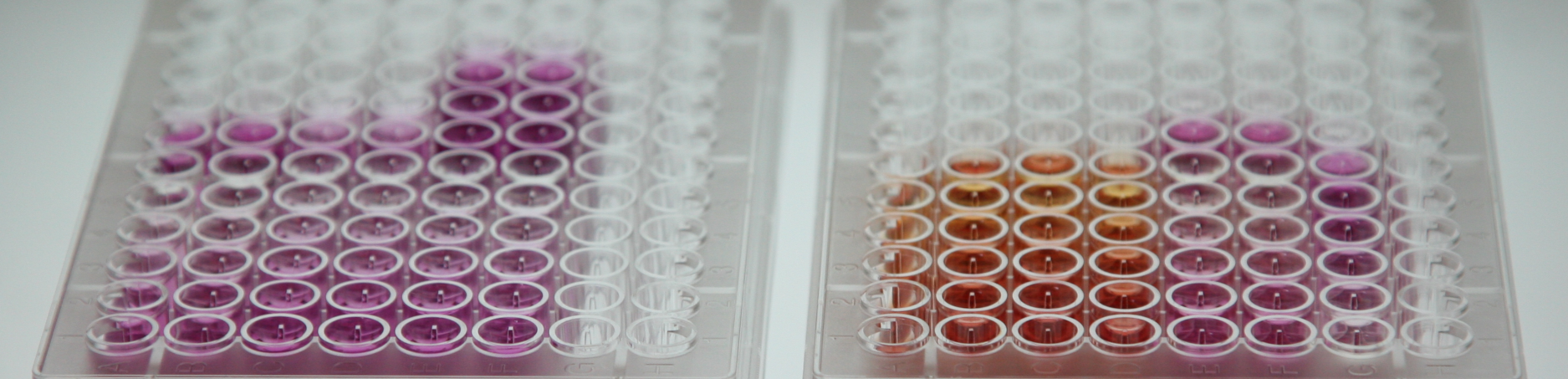
We aim to implement a platform that converges multi-omics and AI to deliver personalized medicine. The platform will involve the surgical resection of meningioma tissue and use of patient-derived xenografts
(PDX). Tissue and PDX will be parallelly evaluated by multi-omics and AI-based KI screening. Multi-omics aid in the identification of pathway dysregulation. AI platform screens for combinations of FDA-approved KIs
Sponsor: Kuni Foundation